Which Element Commonly Has Only a Proton as Its Nucleus
Element X has twelve protons in its nucleus. It has its nucleus and its single proton.
For February It S Iron Atomic No 26
The most common stable form of iodine has an atomic number of 53 protons and an atomic weight of 127 53 protons plus 74 neutrons.
. Hydrogen H is the one and only element in the periodic table containing one proton. Mercury is a chemical element with atomic number 80 which means there are 80 protons in its nucleus. Answer 1 of 7.
Of X 12 and electronic configuration 282. H mass number of 2 b. Soobee72pl and 1 more users found this answer helpful.
It is assigned the chemical symbol U. Neons atomic number is 10 so just multiply 10 by 4 and you get 40. The element is Hydrogen.
A helium B neon C argon D hydrogen E none of the above Answer. As the atomic number of an atom denotes the number of proton as well as the number of electrons in it Hydrogen having the atomic number one and being the very first element in periodic table it has only one proton. The nucleus of an atom consists of.
The atomic number equals the number of protons in the atom. It has a single proton in its nucleus and no neutrons. Answer 1 of 4.
Neon has 10 Protons and four times ten is 40. Which element commonly has only a proton as its nucleus. When the Russian scientist Mendelev first produced his table he was using it to predict what characteristics newly discovered atoms might haveand he.
Determine the number of protons neutrons and electrons in each of the following atoms. Magnesium atoms have two electrons in the outermost shell. The element X belongs to IIA group ie 2nd group since there are only 2 electrons in their valence shell.
Hydrogen H is the one and only element in the periodic table containing one proton. Which element commonly has only a proton as its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol ZThe total electrical charge of the nucleus is therefore Ze where e elementary charge equals to 1602 x 10-19 coulombs.
The isotopes are in the chart of nuclides. An _____ is a pure substance composed of atoms. A uranium atom has 92 protons and 92 electrons of which 6 are valence electrons.
Hydrogen is the only element in entire periodic table with only 1 proton in its nucleus. SolutionThe atomic number of iodine 53 tells us that a neutral iodine atom contains 53 protons in its nucleus and 53 electrons outside its nucleus. Table salt is a compound because it is made up of only one element bound with more than one type of chemical bond.
Al mass number of 27 d. Protons can be found by looking at the atomic number. Which element commonly has only a proton as its nucleus.
Nuclear structure and decay data Hydrogen 6 has 5 times as many neutrons as protons but a very short half-life. To which group of the periodic table it will belong. Cr mass number of 52 c.
Hydrogen atomic number 1 typically only has a single proton in its nucleus. Argon helium hydrogen neon None of the answers is correct. What does the nucleus contain.
2 protons 2 electrons 0 neutrons. As the atomic number of an atom denotes the number of proton as well as the number of electrons in it Hydrogen having the atomic number one and being the very first element in periodic table it has only one proton. So the atomic number gives the protons and the number of.
Uranium is a silvery-white metallic chemical element in the periodic table with atomic number 92. Mercury ²⁰⁴Hg₈₀ has the highest ratio among stable isotopes. The nucleus is the centre.
A singleelectron revolves around in s subshell of. A neon B hydrogen C helium D argon E None of the answers is correct. As a result you would expect magnesium to form ions with a charge of.
What shows an elements number of protons in its nucleus. And Zirconium has 40 protons the symbol for Zirconium is Zr thatgirl562 thatgirl562. Only one element has single protonproton is correct spelling.
As mass number of 75 a. Protons and Neutrons in Mercury. 60 Which element commonly has only a proton as its nucleus.
The whole idea of the periodic table is precisely to show that elements DO have a steady gradient of characteristics. The correct answer is d. Because its nucleus has the correct number of neutrons it is stable and is not radioactive.
The mass of an atom is largely determined by the number of _____ it has. 1 Answer David Drayer Mar 30 2018 the atomic number. Hydrogen has only one proton atomic number 1.
Answer by studying proton collisions hope this. In a neutral atom the number of protons equals the number of electrons.

Publisher Earthscan Uk Homepage Ppt Download

Electricity Chapter 6 Structure Of Atoms Atoms Are Made Of 3 Particles Protons Positively Charges Found In Nucleus Neutrons No Charge Found Ppt Download

Boron Properties Uses Facts Britannica

2012 In Infographics How Graphic News Saw The World Physics Quantum Mechanics Element Chemistry

Things To Learn How Solar Fusion Works Science Humor Science Solar
Chemistry I Atoms And Molecules
The Structure Of The Atom Boundless Chemistry
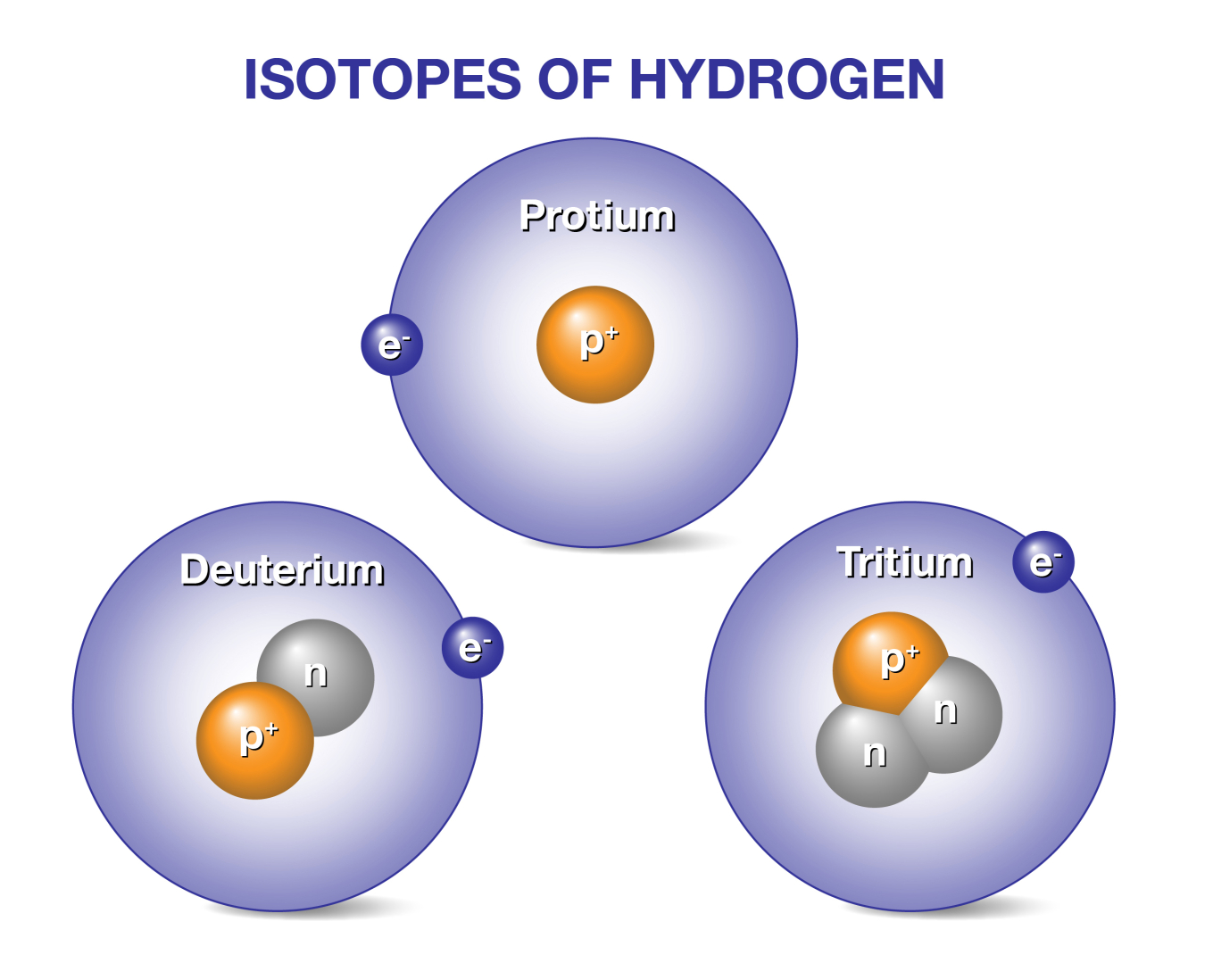
Doe Explains Deuterium Tritium Fusion Reactor Fuel Department Of Energy

Proton Particle In Nucleus With Positive Charge Of 1 And An Atomic Mass Number Of 1 Dalton Neutron No Chemistry Education Electrons Proton Neutron Electron
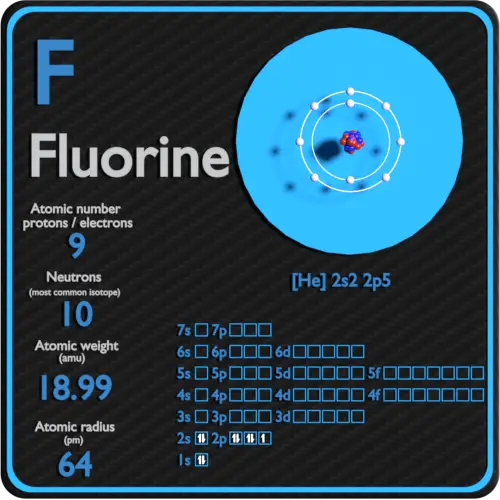
Fluorine Protons Neutrons Electrons Electron Configuration

Review Of Atomic Model You Should Be Able To Ppt Download
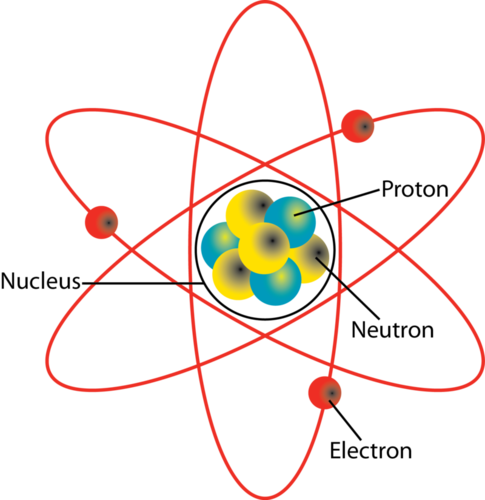
What Two Particles Are Found In The Nucleus Of An Atom Socratic

Beng1113 Principle Of Electrical And Electronics Ppt Video Online Download

How Does The Periodic Table Organize The Elements Ppt Download
Chemistry I Atoms And Molecules

Protons The Proton Is A Subatomic Particle With A Positive Electric Charge Of 1 Elementary Charge Typically Symboli Protons Elementary Particle Science Gifts

Comments
Post a Comment